The Lung Bioengineering and Regeneration (LBR) Lab originated at Lund University in 2017 and since 2024 is located at McGill University. We focus on growing new lung tissue in the lab for transplantation and for use in disease modeling. We use a multidisciplinary approach using advances in materials science (polymer design and synthesis), manufacturing (e.g. 3D bioprinting), and endogenous lung stem cells to construct this bioengineered tissue and these new models. Our work has previously been supported through a Wallenberg Molecular Medicine Fellowship, ERC Starting Grant and through the Swedish Research Council. We are currently supported by a Canadian Excellence Research Chair position in Lung Regenerative Medicine through the Canadian Tri-Agency.
Read more about LBR projects!
I am interested in generating lung tissue in the lab to study disease, develop new therapies and one day to be able to manufacture lung tissue in the lab for transplantation.
I was born and raised in Toledo, Ohio in the United States and received my undergraduate degree in mechanical engineering from Gonzaga University in Spokane, Washington, USA. I conducted my PhD studies in Dr. Sarit Bhaduri’s lab at the University of Toledo where I focused on using microwave processing to generate calcium phosphate nanoparticles for gene therapy and for bone tissue engineering. I went on to do my first postdoctoral training in Dr. Daniel Weiss’ lab at the University of Vermont in the Vermont Lung Center where I was an NIH T32 Fellow and developed protocols to decellularize whole lungs from different species and diseases, including human. I then moved to Munich, Germany to do a second postdoctoral training period in Dr. Melanie Königshoff’s lab at the Comprehensive Pneumology Center (a joint venture between the Ludwig Maximilians University, Helmholtz Center Munich and the Asklepios Clinic). There I focused on the derangement of distal lung epithelial progenitor cells in normal and diseased repair and regeneration.
I started my group ‘Lung Bioenigneering and Regeneration’ in 2017 at Lund University as part of the newly formed Wallenberg Center for Molecular Medicine, with a focus on regenerative medicine. I am currently an Associate Professor and Docent in the Faculty of Medicine at Lund University and head of the Lung Bioengineering and Regeneration Group. I am a Principal Investigator in the Lund Wallenberg Molecular Medicine Center as well as the Lund Stem Cell Center. My lab focuses on generation of lung tissue ex vivo for eventual transplantation and building new humanized models of lung tissue to study chronic diseases and evaluate potential therapies. My work takes a multidisciplinary approach using advances in materials science, manufacturing, and lung stem cell biology to construct these new models. I was selected in 2017 as one of the American Thoracic Society’s ‘Rising Stars of Research’ and was awarded the Carol Basbaum Award by the American Thoracic Society in 2018 – an award given to a young investigator who has demonstrated outstanding scientific achievement, mentorship, and leadership potential. My work is currently funded by the Wallenberg Molecular Medicine Fellowship and a European Research Council Starting Grant in 2018 on 3D bioprinting of lung tissue and a Swedish Research Council Starting Grant in 2018 to pursue mechanisms of repair in chronic lung diseases.
WCMM Highlight Video: “Developing therapies for patients with lung diseases”
Vetenskapens värld episode on Lung Transplantation: “Organ på väg”
PhD in Motor Control
With a PhD in motor control (the neuroscience of movement), I recently transitioned into my role as Program Manager for the Canada Excellence Research Chair (CERC) in Lung Regenerative Medicine (Darcy Wagner). My primary responsibility is to ensure that research projects align with our overarching goals, but I take particular pride in integrating equity, diversity, and inclusion (EDI) principles into every aspect of our work. By doing so, we strive to create a future where cutting-edge medical care is accessible to all.
Master of Science with emphasis in Pathophysiology and Anatomy - FMVZ- USP-Brazil. Specialist in Molecular Biology and Parasitology - IAL-SP. Bachelor of Biological Sciences UAM-SP.
Ambient air pollution accounts for an estimated 4.2 million deaths per year due to stroke, heart disease, lung cancer and chronic respiratory diseases. Around 91% of the world’s population lives in places where air pollution levels exceed WHO limits. Exposure to pollutants contributes to the development of chronic obstructive pulmonary disease COPD is lower than exposure to indoor pollutants. The WHO assumes that only 1% of COPD cases are caused by urban pollution in developed countries and the index in less developed countries goes to 2%. However, there is some evidence linking air pollution with the worsening of COPD, in addition to the fact that recognition of negative health outcomes at high levels of pollution and considering that the airways in turn the lung is the main point of entry for distribution of pollution particles to the rest of the body, since ultrafine particles are found in various organ.
The presence of air pollution is not only related to COPD but also inflammation, development of COPD, increased risk of Alzheimer’s development, and other adverse health effects including effects on fetal development through exposure of pregnant women to areas with high concentrations of pollutants. For this purpose, the evaluation of the regenerative capacity of lungs exposed or not to high concentrations of suspended material in the air is important.
The central goal of this project is to investigate where these particles end up in the lung, what they are, and how they affect lung regeneration capacity.
Bioengineering Undergraduate Student
I am a fourth-year bioengineering student at McGill University with a broad interest in the intersection of engineering and biology, whether that be in tissue engineering, environmental engineering, life-sustaining systems in space, or conservation research. I just completed the SURE (Summer Undergraduate Research Experience) program at the environmental engineering lab at McGill, where I worked on the design of primers for near full-length amplification and characterization of antimicrobial resistance genes in wastewater and drinking water in the Montérégie area of Quebec. I am continuing my work part-time at this lab. I am also involved in the payload subteam of the McGill Rocket Team, where I have worked on an experiment to test pull-out strength of spinal pedicle screws to assess their potential in preventing screw micromotion during rocket flight.
I am currently working on my engineering capstone project at the LBR lab where I look forward to broadening my knowledge and experience with tissue and cell culture engineering. My team and I are focusing on the design of an open-source 3D bioprinter with multi-extrusion capabilities to produce complex, multicellular structures appropriate for transplantation.
Bioengineering student
I am Berta, a fourth-year Biomedical Engineering student, and I am passionate about combining engineering with medicine to develop innovative solutions that improve people’s health and quality of life. I am particularly interested in the field of regenerative medicine and tissue engineering, as they hold enormous potential to transform the future of biomedicine.
My current project focuses on the development of collagen hydrogels and how their self-assembly process can be influenced by changes in the ionic environment. The goal is to understand how these variations affect their structural and functional properties, and thus explore their potential as scaffolds for tissue engineering. With this knowledge, I aim to design advanced biomaterials inspired by the extracellular matrix, to be applied in innovative techniques such as bioprinting and cell encapsulation.
Bioengineering student at McGill University
I’m a fourth-year bioengineering student at McGill University. Last year, I was a wet lab member of the McGill iGEM team where I worked on nucle.io, a project leveraging DNA computing to diagnose sepsis. I am now part of the payload project for Rocket Team.
I recently had the opportunity to study abroad in Bologna, Italy, where I broadened my academic horizons. This experience strengthened my adaptability, cross-cultural communication skills, and ability to approach problems from multiple disciplinary perspectives.
I’m Milana, an undergraduate student in Bioengineering at McGill University. I am passionate about tissue engineering, regenerative medicine, and bioprinting. I believe these fields can help solve some of the biggest challenges in medicine, like organ shortage.
At the Lung Bioengineering and Regeneration Lab, I have worked on transforming an open-source 3D printer into a bioprinter for lung tissue applications, including performing technical evaluations and designing custom components. My next project, as part of my engineering capstone, will involve completing the development and design of this printer, turning it into a multi-extrusion 3D bioprinter capable of producing complex, multicellular constructs such as miniaturized lung airways for transplantation. The goal of this work is to create accessible, customizable, and low-cost bioprinting tools that can democratize research in tissue engineering and regenerative medicine.
I am a Medical Doctor graduated from Tbilisi State Medical University,Georgia. My passion for learning basic molecular mechanisms behind disease development and pathogenesis led me to join the Lung Bioengineering and Regeneration laboratory at Lund University as a research engineer.Currently I am involved in multidisciplinary projects such as the decellularization of different tissues, the establishment of different ARDS models in large animals and a drug screening project.
I am Osandi, an undergraduate student in Bioengineering at McGill University. I am interested in the intersection of cancer biology, tissue engineering, and mechanobiology, and enjoy working on research projects that integrate engineering principles with complex biological systems.
My previous research focused on the mechanobiology of prostate cancer, examining how androgen receptor signaling and microenvironment stiffness influence cell behavior and metastatic progression. This experience deepened my understanding of how mechanical cues regulate cellular behavior and continues to guide my approach to engineering biomimetic tissue environments.
For my engineering capstone project, I am working at the Lung Bioengineering and Regeneration lab (LBR) at the RI-MUHC to develop an open-source, multi-extrusion 3D bioprinter capable of fabricating multicellular, miniaturized tissue constructs. This project aims to overcome the limitations of commercial bioprinters and enable more physiologically relevant lung tissue models for advanced research.
Generating Hydrogels for Regenerative Medicine Applications
A Master of Science (MSc) Candidate in Experimental Medicine, specializing in Lung Regenerative Medicine. My research focuses on developing innovative therapies for lung tissue repair and regeneration, using approaches like stem cell therapy, tissue and biomaterial engineering, and 3D cell culture🧫.
With a background in molecular biology and translational research, I am dedicated to advancing regenerative strategies for lung diseases. Deeply passionate about applying scientific discoveries to real-world healthcare challenges, I’m eager to connect with professionals in the fields of regenerative medicine and respiratory health🔬🫁.

Advancing Regenerative Medicine through clinical translation and GMP excellence.
I am a Medical Doctor and Pharmacist with postgraduate training in Pharmaceutical Research and Development, and over a decade of experience spanning R&D, Quality Assurance, Regulatory Affairs, and Medical Affairs in the pharmaceutical industry. My professional journey took a transformative turn when I encountered Regenerative Medicine, a field where I discovered my true passion.
At the LBR, where we are developing advanced therapeutic products, I bring my expertise to support the clinical translation of these innovations, a fundamental step in making cell and gene therapies not only available but also truly accessible to patients in need. I am particularly committed to ensuring that every stage of this process is conducted in accordance with Good Manufacturing Practices (GMP), thereby safeguarding patient welfare while meeting the highest regulatory expectations.
I am Letizia, an undergraduate student in Bioengineering at McGill University. I am driven by curiosity and love working in multidisciplinary environments where engineering meets biology. At the Lung Bioengineering and Regeneration lab (LBR) at the RI-MUHC, I have been exploring different aspects of tissue engineering and 3D bioprinting through several hands-on research projects.
My first project focused on optimizing protocols for hydrogels derived from decellularized lung extracellular matrix (dECM), with the goal of creating bioinks to 3D bioprint lung tissue and constructs. I am currently developing a 3D bioprinted model of breast cancer metastasis in the lungs to help better understand tumor progression in physiologically relevant models. My next project, as part of my engineering capstone, will involve designing and building a multi-extrusion 3D bioprinter capable of printing complex tissue structures, such as small airways.
“Regeneration reveals the hidden potential within tissues, turning the challenges of disease into opportunities for healing” I’m Alfredo Moreno Olmedo, a PhD candidate in Experimental Medicine at McGill University, with a focus on lung tissue regeneration in lung disease. Before starting my PhD, I completed a specialization in Bioethics. I hold an MD from the National Autonomous University of Mexico (UNAM) and an MSc in Stem Cells and Regenerative Medicine from the University of Sheffield in the United Kingdom, where I worked on microporous 3D scaffolds for musculoskeletal tissue engineering. My interest in regenerative medicine began in Xochimilco, my hometown in Mexico City (“where the flowers grow”), inspired by the extraordinary regenerative abilities of the axolotl. This experience motivated me to explore ways to restore damaged tissues, going beyond conventional medical approaches. I attended the CAS-ANSO Summer Camp at the University of the Chinese Academy of Sciences (UCAS) in Beijing, collaborating with researchers from diverse backgrounds. I also founded Neotissues, a startup dedicated to promoting knowledge in regenerative medicine, connecting experts, and making science more accessible to the public. I am committed to advancing biomedical research while fostering global collaboration and sharing knowledge. I enjoy learning new techniques, exchanging ideas, and working with others to bridge the gap between discovery and real-world applications.
Shahram completed his PhD in Immunology at the Department of Dermatology and allergology of Szeged University, Hungary. His doctoral research focused on cancer biology, investigating the effects of bile acids on pancreatic cancer tumorigenesis and developing a 3D bioprinted tumor model to study the role of adipose-derived mesenchymal stem cells (ADMSCs) in tumor microenvironment–driven chemoresistance. He integrated transcriptomic and proteomic approaches to uncover the molecular mechanisms underlying therapy resistance and tumor–stroma interactions.
Before his PhD, Shahram earned a BSc in Clinical Laboratory Sciences and an MSc in Medical Immunology. He has broad experience in protein purification, immunochemistry, bioconjugation, biomaterial-based tissue engineering, and stem cell biology across both academic and industrial settings.
Since 2023, Shahram has been working as a research engineer in the Lung Bioengineering and Regeneration (LBR) Group at Lund University. He is currently an IGNITE Fellow in the Wagner Lab, where he is developing a humanized airway organoid model to study Pseudomonas aeruginosa, a WHO priority pathogen that urgently requires new therapeutic strategies. His project involves creating apical-out airway organoids derived from induced pluripotent stem cells (iPSCs) to mimic physiological infection routes and integrating microfluidic systems to enable long-term co-culture with live bacteria.
Willing to tackle global health issues through research and innovation in regenerative medicine.
Currently a Postdoctoral Fellow at McGill University, working at the Meakins-Christie Laboratories within the RI-MUHC. My research focuses on using cell-derived extracellular matrix and iPSC-derived cells to develop new approaches for lung tissue regeneration.
During my PhD studies in Dr. L’Heureux’s lab at the University of Bordeaux, I focused on developing completely biological tissue-engineered vascular grafts and led a pre-clinical study in a large animal model. I went on a first postdoctoral position in Dr. Elliot Chaikof’ lab at Harvard Medical School, where I developped biomimetic ECM-based scaffolds and studied the use of human iPSC-derived cells for vascular regeneration.
Beyond laboratory research, I have a strong interest in innovation and translating lab findings from the bench to bedside, which led me to pursue a Master’s degree in Biomedical Entrepreneurship.
Microscopy unveils the hidden architecture of the microscopic world, turning the invisible into a universe of breathtaking detail.
I’m Leonor, a PhD candidate at McGill University, working at the Meakins-Christie Laboratories within the RI-MUHC. My research builds on my bachelor’s thesis, focusing on light-sheet fluorescence microscopy (LSFM) and the protocols required for processing samples with this advanced imaging technology. Microscopy and histology have long been passions of mine, ones that have only deepened as I’ve immersed myself further in the scientific world.
Beyond imaging, I have a strong interest in Regenerative Medicine, which led me to pursue my master’s degree in the field. During my PhD, I will also work on translating lung regeneration research into real-world applications, bridging the gap between discovery and clinical impact.
I am always eager to learn new techniques and expand my skill set. Equally important to me is sharing knowledge and fostering collaboration. I enjoy making others part of my work, whether by exchanging ideas, discussing methodologies, or guiding them through the techniques I use.
Biomedical scientist-to-be with a clinical backbone
Hi, my name is Indra. I am a Master in Biomedicine student at Lund University and originally from Indonesia. Within the Lung Bioengineering and Regeneration Lab, I am working on my master degree project on developing a high-throughput lung ALI culture-based platform from primary human bronchial basal cells for COVID-19 prophylaxis drug screening. With my basic as medical doctor, I am naturally enticed with basic researches that is leaning on the translational side and this platform will hopefully be useful not only for the purpose of COVID-19 drug screening, but also for general use in high-throughput lung-related researches. In my free time I love to do music and I am singing together with Lunds Studentsångare as a Tenor.
Darcy Wagner
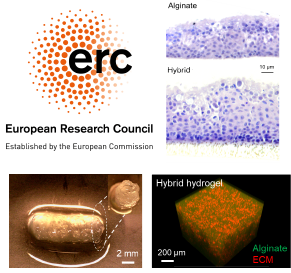
Many people with end-stage lung disease have no other option than to undergo lung transplantation. There are, however, not enough donor lungs to meet clinical demand, heightening the need for new options that can increase available tissue for lung transplantation. The EU-funded 3DBIOLUNG project will focus on generating lung tissue in the lab using bioengineering approaches and explore whether new advances in 3D printing and 3D bioprinting can be a possible option for lung tissue for transplantation. To do this, it will use custom 3D bioprinters to generate constructs imitating lung tissue, 3D bioprint hybrid murine and human lung tissue models, and test gas exchange, angiogenesis and in vivo immune responses.
John Stegmayr
Darcy Wagner
Sandra Lindstedt (Region Skåne, Lund University)
Lena Uller (Lund University)
In this project, we aim to develop and validate cryopreservation methods for human PCLS for use in ultimately studying SARS-CoV2 infection and evaluation of potential therapies. The development of cryopreservation techniques would allow many more experiments to be possible from a single human lung, but would also allow for the establishment of a biobank of cryopreserved PCLS (that could be used on-demand and shipped worldwide). Cryopreserved PCLS would allow testing of SARS-CoV2 in labs with access to the virus and appropriate BSL3 facilities. If fully realized, the infection model could then be run using a library of compounds with FDA/EMA approval for repurposing. As an alternative to the infection model, there has recently been reports of characterizing the late stages of infection, including characterization of the cytokine profile of patients who succumb versus those who survive. Our work previously used this approach of simultaneous exposure to multiple cytokines to develop an ex vivo model of fibrosis (Alsafadi et al. AJP-Lung 2017). This approach could be explored in parallel to identify drugs to combat lethal SARS.
Sinem Tas
Emil Rehnberg
Darcy Wagner
As the COVID-19 pandemic progresses, it has become increasingly clear that SARS-CoV-2 disproportionately effects different patient populations and that age and underlying co-morbidities are major predictors of mortality. In parallel, it is now well acknowledged that the general population is susceptible to infection with SARS-CoV-2, but most patients remain asymptomatic and do not develop severe disease. Several potential vaccines have reached late phases of clinical trials, but even if a successful vaccine is identified soon, concerns will remain with administering a vaccine to the world population and technologies to ramp up production are still needed. During this time, it is critical to identify approaches to protect vulnerable patient populations so that society can begin to return to near normal. One potential option is to repurpose existing antiviral compounds which can prevent infection in vulnerable patients. Here, we will perform a medium throughput drug screen in primary human airway cells, a major site of SARS-CoV-2 infection, to identify approved antiviral compounds which could be repurposed for prophylactic SARS-CoV-2 therapy in vulnerable populations. Together with Cellevate AB, we have developed a new method for medium-high throughput drug screening of human airway cells which allows several hundred compounds to be evaluated on cells from individual patients. Furthermore, this assay is amenable to validation of compounds in human airway cells derived from patients with underlying co-morbidities associated with COVID-19. This approach has the potential to identify and repurpose antiviral compounds with previously established safety profiles to protect vulnerable patients until a vaccine is developed.
Contact Us





















LBR on the news
Darcy Wagner receives prestigious JPND award
RI-MUHC 2025 news
PhD defence interview – Martina De Santis
LU Med News 2021
A new interdisciplinary co-op between researchers, healthcare and industry formed to fight the virus
LU Med News 2021
Kliniskt fysiologisk lungforskning
Lung & Allergiforum 2020
Meet this week’s Wallenberg Researcher: Darcy Wagner
LU Med News 2020
New Promising Treatment Uses Smart Nanoparticles to Target Lung Cancer
LU Med News 2020
Här skapas lungvävnad på laboratoriet
Forskning & Framsteg 2019
Darcy Wagner Receives ERC starting grant
LU Med News 2018
Four LU researchers receive ERC starting grants
LU News 2018